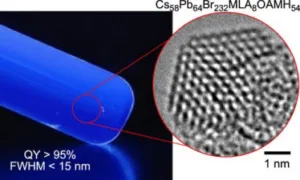
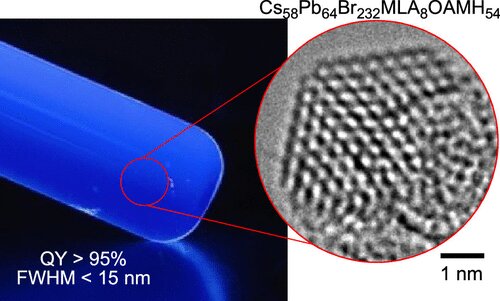
Most quantum dot displays use a source of blue light (LEDs in nearly all cases except Samsung Display’s QD-OLED panels) but this means that you have to make the blue light emit from the display in the same way as the red and green light, which adds some challenges.

In particular, more than you want of the blue light can ‘leak through’ the red and green layers, diluting the colour purity and making it hard for display makers to make the wide colour gamut displays that buyers want. One way to solve this is to have thicker layers of red and green dots, but that means more QDs and they can be expensive.
Another way to avoid the problem is to use UV light, which is invisible to humans, as the energy source and generate not only the red and green using quantum dots, but also the blue. This is an approach that Applied Materials is using to try to develop microLEDs (Applied Materials Overcomes Some Challenges in MicroLED for VR). However, blue quantum dots are not easy to make or, currently, widely available. They need very tightly controlled physical parameters to convert to blue.
A New Approach From Tokyo
A group from the University of Tokyo’s Department of Chemistry has tried a new approach and reports (in the journal of the American Chemical Society ) that it has made QDs that can convert UV light with a wavelength of ?460 nm with a narrow half-maximum linewidth below 15 nm. Rec 2020 specifies a 467nm blue primary. The research group has used a different approach to others that have found and created molecules ‘top down’ and describes that it made the quantum dots ‘bottom up’. Professor Eiichi Nakamura from the University told Optics.org:
“Previous design strategies for blue quantum dots were very top down, taking relatively large chemical substances and putting them through a series of processes to refine them into something that worked. Our strategy is bottom up. We built on our team’s knowledge of self-organizing chemistry to precisely control molecules until they form the structures we want. Think of it like building a house from bricks rather than carving one from stone. It’s much easier to be precise, design the way you want, and is more efficient and cost effective too.”
 Electron microscope images of experimental samples using different chemical combinations. Credit: ©2022 Nakamura et al.The group used a a hybrid mix of including lead perovskite, malic acid and oleylamine and these materials are coaxed into the form required, which is a structurally uniform cube of 64 lead atoms, four to a side. Eight malate molecules are located on the eight corners of the cubes, and oleylammonium cations lipophilize and stabilize the edges and faces. The group sees it as a molecule rather than a nanocrystal.
Electron microscope images of experimental samples using different chemical combinations. Credit: ©2022 Nakamura et al.The group used a a hybrid mix of including lead perovskite, malic acid and oleylamine and these materials are coaxed into the form required, which is a structurally uniform cube of 64 lead atoms, four to a side. Eight malate molecules are located on the eight corners of the cubes, and oleylammonium cations lipophilize and stabilize the edges and faces. The group sees it as a molecule rather than a nanocrystal.
Malic Acid a Key
The group spent a year trying different materials until it found that malic acid was a key part of the recipe – that was one of its biggest challenges. Also because the QD is 290 times smaller than a wavelength of blue light, at 2.4nm, it was difficult to view. The team used a technique that it pioneered of SMART-EM, or ‘cinematic chemistry’. That is a video-like technique and can be seen on video here. There are also a number of videos here.
The group said that as well as being photoluminescent, the materials are electroluminescent so might also be developed for the next generation of ELQD displays.
Now, an industry colleague, years ago, said to me “Don’t believe it about blue materials unless you hear the colour, the lifetime and the efficiency, all at the same time”. That was in connection with OLEDs, but it often applies as achieving better blue can kill the efficiency or higher efficiency can kill the lifetime – there are always trade-offs. In the case of this material, which is an R&D project, not at the industrialisation phase is that the materials have a very short life time. The group said that it is in discussions about how to extend the lifetime and industrialising the material. (BR)
Precision Synthesis and Atomistic Analysis of Deep-Blue Cubic Quantum Dots Made via Self-Organization
Olivier J. G. L. Chevalier, Takayuki Nakamuro, Wataru Sato, Satoru Miyashita, Takayuki Chiba, Junji Kido, Rui Shang and Eiichi Nakamura