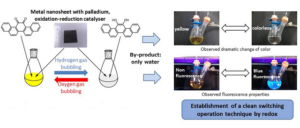
Researchers from Kumamoto, Yamaguchi and Osaka Universities in Japan have developed a method of changing the colour and fluorescence of compounds, using oxygen (O2) and hydrogen (H2).
The (reversible) reaction produces water as a byproduct and is so said to be environmentally friendly. Energy from the O2 and H2 gases themselves is used to fuel the process. The researchers say that the method could be used as a detection sensor for H2 or O2 gases, or for property controls of organic materials: for instance, turning an OLED on or off.
Research at Kumamoto University focused on using energy from gases to trigger a molecular switch in a polyaromatic compound (PAC). Specifically, the focus was on using H2 as a reductant and O2 as an oxidant. H2 gas was bubbled into a solution made of synthesised picene-13, 14-dione and a palladium nanoparticle catalyst. This had the effect of changing the colour from yellow to colourless and the fluorescence from non-fluorescent to fluorescent blue. Exchanging the H2 gas for O2 reversed the process.