Mobile – Batteries are the life blood of mobile electronic devices. There is, therefore, an ongoing desire for rechargeable batteries with increased energy storage capacity and that can be more rapidly recharged. A recent and potentially significant development in battery technology has been reported by a team within the School of Materials Science and Engineering at the Nanyang Technological University (Singapore).
The science behind the new battery was reported in an article entitled “Mechanical Force-driven Growth of Elongated Bending TiO2-based Nanotubular Materials for Ultrafast Rechargeable Lithium-ion Batteries” published in Advanced Materials 2014, 26, 6111-6118. The article is available for purchase on-line and can be found here.
One observation that can be made before discussing any details of the new battery technology is to characterize it as an evolutionary improvement upon existing lithium-ion battery technology. Conventional lithium-ion batteries usually use additives to bind the electrodes to the anode (negative pole). This affects the speed by which electrons and ions can transfer in and out of the battery. The new method eliminates the need for these additives and enables the packing of more energy into the same volume.
The basis of the new approach is the use of nanostructures. Instead of graphite, the material traditionally used to create the anode of a lithium-ion battery, the new technology uses a titanium dioxide gel. This “raw material” is both abundant and environmentally safe.
Naturally occurring in a spherical shape, the team developed a simple method to turn titanium dioxide particles into tiny, ultralong, nanotubes. The nanotubes are used to produce an electrode described as based on a three dimensional, cross-linked network of titanium dioxide nanotubes.
Team leader, Associate Professor Chen Xiaodong, explains that producing the nanotube gel requires, in part, mixing together titanium dioxide and sodium hydroxide and then stirring at a certain temperature.
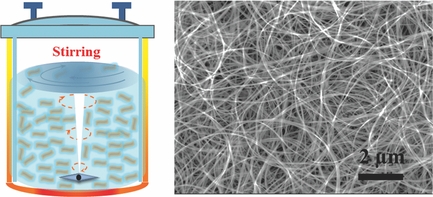
Xiaodong goes on to state that manufacturing the titanium nanotubes should be quite easy. The team further believes that battery manufacturers will not find it difficult to integrate the new gel into current production processes.
The resulting electrode exhibits a “capacitor-like rate performance and a battery-like high capacity for long-time cycling”. These characteristics are attributed to the pseudocapacitive charge storage process, short diffusion length, large surface area as well as the reduced electron conductivity of the elongated nanotube electrode.
Testing has shown that the electrode can cycle over 10,000 times in half cells. This is 20 times more than the 500 cycle capability of today’s batteries. At the same time, the new battery can still retain a relatively high capacity of 114 mA h/g. The nanostructure also speeds up the chemical reactions that take place within the battery. This allows for charging at an ultra-high rate at 25 C of 8.4 A/g.
Since the new batteries can last ten times longer than the current generation of lithium-ion batteries, users can save on the cost of a battery replacement. Equally important, it is possible to drastically cut down the waste generated by the need for the disposal of dead batteries.
Chen’s research team is in the process of applying for funding to fabricate a large-scale battery prototype. The technology is currently being licensed to a company and Chen anticipates that the new fast-charging batteries will be commercially available in about two years. – Arthur Berman
Nanyang Technological University, Chen Xiaodong, +65-6513-7350, [email protected]

