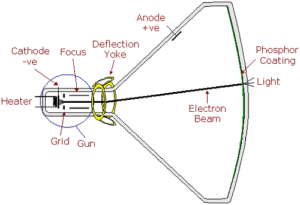
The control grid regulates the number of electrons which are allowed to leave the gun of a CRT by using a negative voltage to repel the electrons. Once free of the control grid’s influence, the electrons then need to be accelerated down the tube to ensure that they strike the phosphor face-plate lining with sufficient energy to excite the phosphor molecules and produce light.
Just as a negative voltage repels the electrons, a positive voltage will attract them and an electrode, called the anode, to which a positive voltage is applied is used to provide this acceleration. The process is similar to that used to accelerate space probes by making use of the gravity-well of a planet to provide a “sling-shot” effect. The idea being that, by the time the electrodes arrive in the vicinity of the anode, they are going too fast to be sufficiently diverted from their path to strike it.
In the Braun tube the anode was mounted in the neck of the tube but later experimenters found that locating the anode closer to the faceplate, and operating it at very high voltages, was more effective. In modern CRTs, therefore, the anode is located in the funnel portion of the tube and takes the form of a metal coating or foil applied to the inside of the tube. In aluminised tubes, the aluminium coating itself is used as the anode.
Incidentally, the terms electrode, cathode and anode were coined by Michael Faraday over 150 years ago to describe elements in the process of electrolysis.