Actually, the announcement by E Ink says, Harvard study shows E Ink’s ePaper is Up to three times healthier for your eyes than LCD screens. The paper is co-written by four researchers, two of whom are, Dr. Dirk Hertel, principle scientist at E Ink, and Lynne C. Garone, associate vice president at E Ink. It’s lab-based experiment that uses retinal cells to measure the effect of various devices on the mitochondria of the cells. It’s a lot of shining light on petri dishes in a lab.
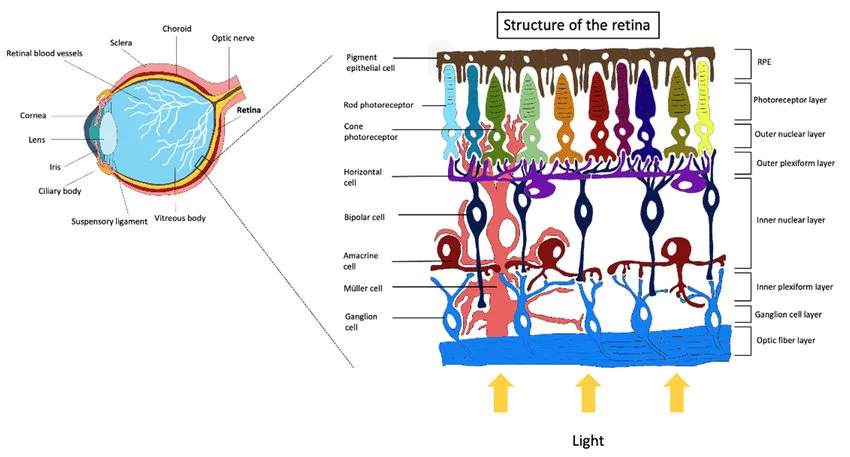
The study investigates the effects of different handheld displays on basic cellular functions. The specific aim is to determine whether direct exposure to the spectral emissions from these devices can trigger measurable biological effects in a controlled environment. ARPE-19 cells were cultured and maintained in DMEM: F-12 Medium supplemented with 10% fetal bovine serum at 37 °C in a humidified 5% CO2 incubator. To test cell viability, cells were released from the tissue culture substrate and counted using a Countess II FL automated cell counter. Cell plates were irradiated by the screens of each study device to mimic retinal irradiance, with exposure times set at 30, 60, 120, and 240 min. ROS expression levels were measured immediately following exposures from study devices at the four exposure times using ROS-Glo H2O2 luminescence assay. Mitochondrial morphology was analyzed by staining cells with MitoTracker Fluorescence dye (specifically labels mitochondria) and imaging them using a confocal laser scanning microscope. The images were analyzed using arivis Scientific Imaging Platform to obtain measurements of mitochondria circularity and interconnectivity. From 10 to 30 observations were made of mitochondrial morphologies from different cells depending on the sample. The results were used for qualitative analysis only.
The study devices listed in the table below represent a sample of currently available handheld displays that are widely used for prolonged periods of reading. The devices use different display technologies, each with LED illumination of various spectral outputs.
The passage also notes that Devices 1, 2, and 3 have user-controlled options for day and night modes that change the color of the display light from cold white for daytime viewing to a warm white with less blue light for nighttime viewing. This is relevant because blue light has been shown to have a more significant impact on sleep and circadian rhythms than other types of light, and it is possible that prolonged exposure to blue light could have negative effects on cellular function.
Device 3 is also noted to have an experimental frontlight that differs from the others by using LEDs with the blue emission shifted to 460 nm, away from the peak of the blue-light hazard function. This suggests that the researchers are taking specific steps to minimize the potential harm caused by blue light exposure.
| Sample ID | Day and night modes | White screen color | White screen luminance (cd/m2) | Display technology | Display lighting | BLTF < 0.085 | CCT (Kelvin) |
| Device 1D | Day | Cold white | 423 | Color LCD | Backlight | 0.087 | 6691 |
| Device 1N | Night | Warm white | 285 | Color LCD | Backlight | 0.025 | 2763 |
| Device 2D | Day | Cold white | 116 | B&W EPD | Frontlight | 0.091 | 7370 |
| Device 2N | Night | Warm white | 89 | B&W EPD | Frontlight | 0.011 | 2284 |
| Device 3D | Day | Cold white | 91 | Color EPD | N/A | 0.061 | 4741 |
| Device 3N | Night | Warm white | 89 | Color EPD | N/A | 0.037 | 3306 |
| Device 4D | Day | Cold white | 88 | Color EPD | N/A | 0.081 | 6462 |
In terms of how these findings relate to real-world scenarios, it is important to note that this study was conducted in a controlled laboratory environment and the findings may not perfectly translate to how handheld devices impact biological systems in the real world. However, the study provides valuable insights into the potential biological effects of prolonged exposure to the spectral emissions from handheld displays.
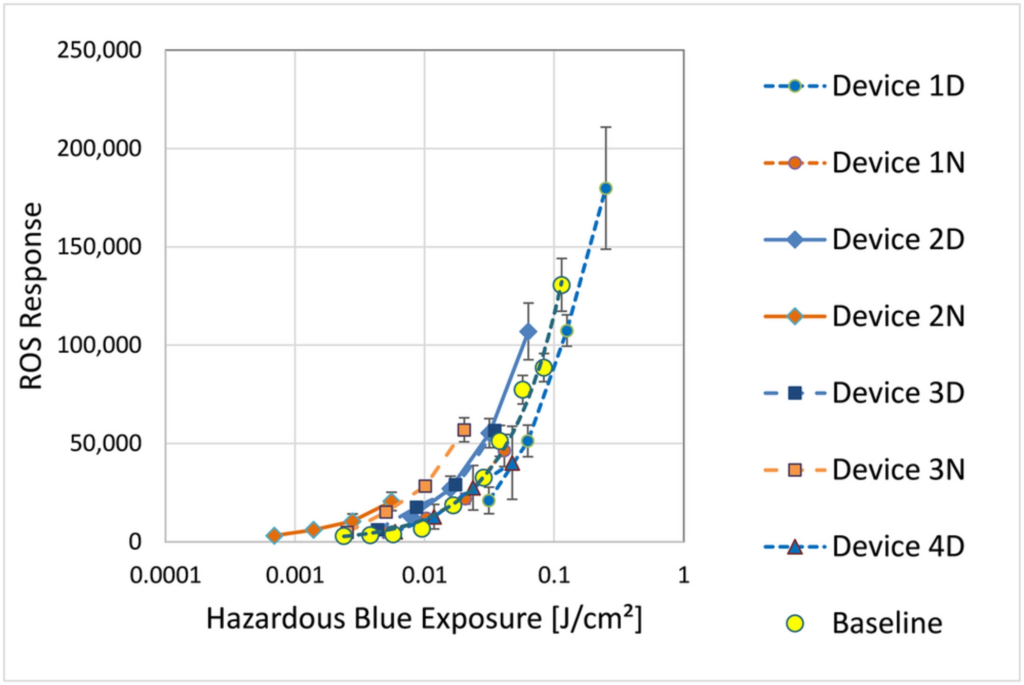
The conclusion of the experiment indicates that the exposure of in vitro human retinal epithelial cells to light from tablet-sized displays can trigger a cellular response, including the accumulation of ROS (reactive oxygen species) and changes in mitochondrial morphology. The study finds that the ROS response to light exposure from different devices generally increased with time, but differences in the device spectra caused different ROS responses to the same hazardous blue exposures.
Furthermore, the study found that cells accumulated ROS two to three times as fast when being exposed to backlit LCD compared to frontlit EPD, each operated in the same light mode (day or night). This suggests that devices that cause ROS accumulation at a lower rate, such as EPD with frontlight, may be used for longer times before the same levels of ROS are reached.
The authors also note that different relative amounts of red light in the device spectra may mitigate the effects of hazardous blue exposure, but this requires further study to confirm and quantify such a protective effect.
Studying the effects of handheld devices on personal health is a complex and multifaceted issue that requires a comprehensive approach. While in vitro studies like the one described here can provide insights into the potential biological effects of exposure to certain types of light emitted by electronic devices, they are limited in their ability to fully capture the complexities of real-world scenarios.
Reference
Wang, X, Hertel, D, Garone, LC, Rogers, RA. Comparison of oxidative stress response of in vitro retinal cells exposed to blue light from emissive versus reflective displays. J Soc Inf Display. 2023; 31( 3): 112– 124. https://doi.org/10.1002/jsid.1191