Chemists at MIT have developed a new method to synthesize stable, color-tunable organic molecules known as acenes. The innovation addresses a key barrier to wider use of acenes in optoelectronics.
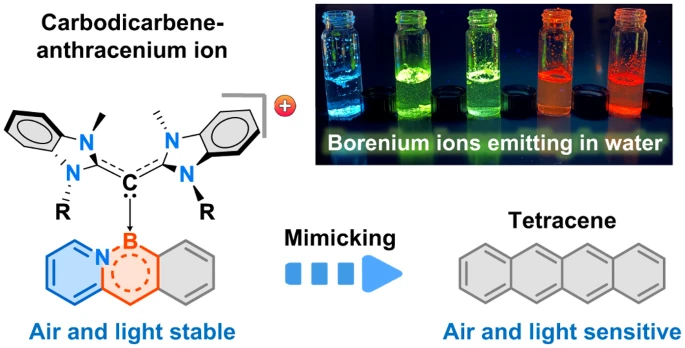
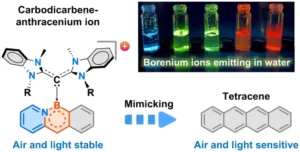
Acenes show promise in OLEDs, thin-film transistors, solar cells, biological imaging and other applications. However, longer acenes tend to decompose when exposed to air or light. The new MIT technique gets around this by attaching ligands called carbodicarbenes to the acene backbone.
“With the addition of carbodicarbenes, the acenes became positively charged, which improved their stability,” said Dr. Robert Gilliard, senior author of the study. “We created red, orange, yellow, green and blue emitters, which is important for diverse applications.”
For example, red fluorophores are needed for biological imaging because many tissues emit blue light. The new acenes also remain stable in water, unlike similar compounds, further expanding their possible medical uses.
In addition, Dr. Gilliard plans to collaborate with MIT electrical engineering professor Marc Baldo on incorporating the acenes into highly efficient solar cells that can produce two electrons from one photon.
“We’re still in the early stages of developing specific applications,” Dr. Gilliard said. “But due to their enhanced stability, device fabrication should be much smoother.”
Reference
Deng, CL., Obi, A.D., Tra, B.Y.E. et al. Air- and photo-stable luminescent carbodicarbene-azaboraacenium ions. Nat. Chem. (2023). https://doi.org/10.1038/s41557-023-01381-0