A team of researchers have synthesized fluorescent compounds based on a so-called “merry-go-round” molecular complex. These molecules have the potential to be used in OLEDs that are brighter and more economical to produce than ones based on conventional polymers.

The team was headed by Alexander Smol’yakov of RUDN University (Moscow, Russia) along with colleagues from the A. N. Nesmeyanov Institute of Organoelement Compounds within the Russian Academy of Science (Moscow, Russia) and the Saint Petersburg State University
A recent article on this topic by the team is entitled “Luminescent Complexes of the Trinuclear Silver(I) and Copper(I) Pyrazolates Supported with Bis(diphenylphosphino)methane.” The article was published in Inorg. Chem. 2019, 58, 13, 8645-8656. A copy of the article is available for purchase here.
First, a few words of background information.
The fabrication of conventional OLEDs typically includes the use of expensive, conductive polymers. In addition, conventional OLED materials are typically quite toxic thus creating challenges during production and problems related to waste disposal. One approach that has been investigated to reduce the extent of these problems is the use of fluorescent complex compounds. Such molecules include small organic fragments surrounding a central ion which is a metal rather than a polymer. Unfortunately, up till now, none of these complexes have been found to demonstrate a clear advantage in brightness and efficiency over polymers. The trade-offs are that effective compounds can be produced based on iridium or platinum but are expensive. Less expensive compounds can be produced based on transition metal ions but are less effective.
The approach adopted by the team to address these issues is the synthesis of molecules having a nucleus that is a triangle of monovalent copper or silver atoms. The organic elements are bound to the nucleus through phosphorus atoms that rotate around them. These molecules proved to be more effective and less toxic compared to polymers. As a separate matter, they should also be less expensive to fabricate.
An interesting if somewhat technical discussion of the how and why the rotation occurs is included in the article and can be paraphrased as follows: within the center of the complex there are two phosphorus atoms and three metal atoms. As a result, one of the metal atoms is always without a phosphorus partner. At room temperature, the energy of thermal oscillations is sufficient to momentarily break the bond between phosphorus and metal atoms. If/when there is a momentarily unattached phosphorus atom, it is immediately attracted to the unpatrnered metal atom – that is, the phosphorus atom “jumps” to the neighboring metal atom where a bond is formed that can once again be broken by thermal fluctuations.
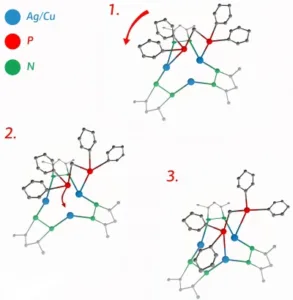
Described in more colorful if somewhat simplistic terms, “the molecule thus turns into a kind of molecular merry-go-round.” The three configurations that can be adopted by such a molecule are illustrated in the figure below.
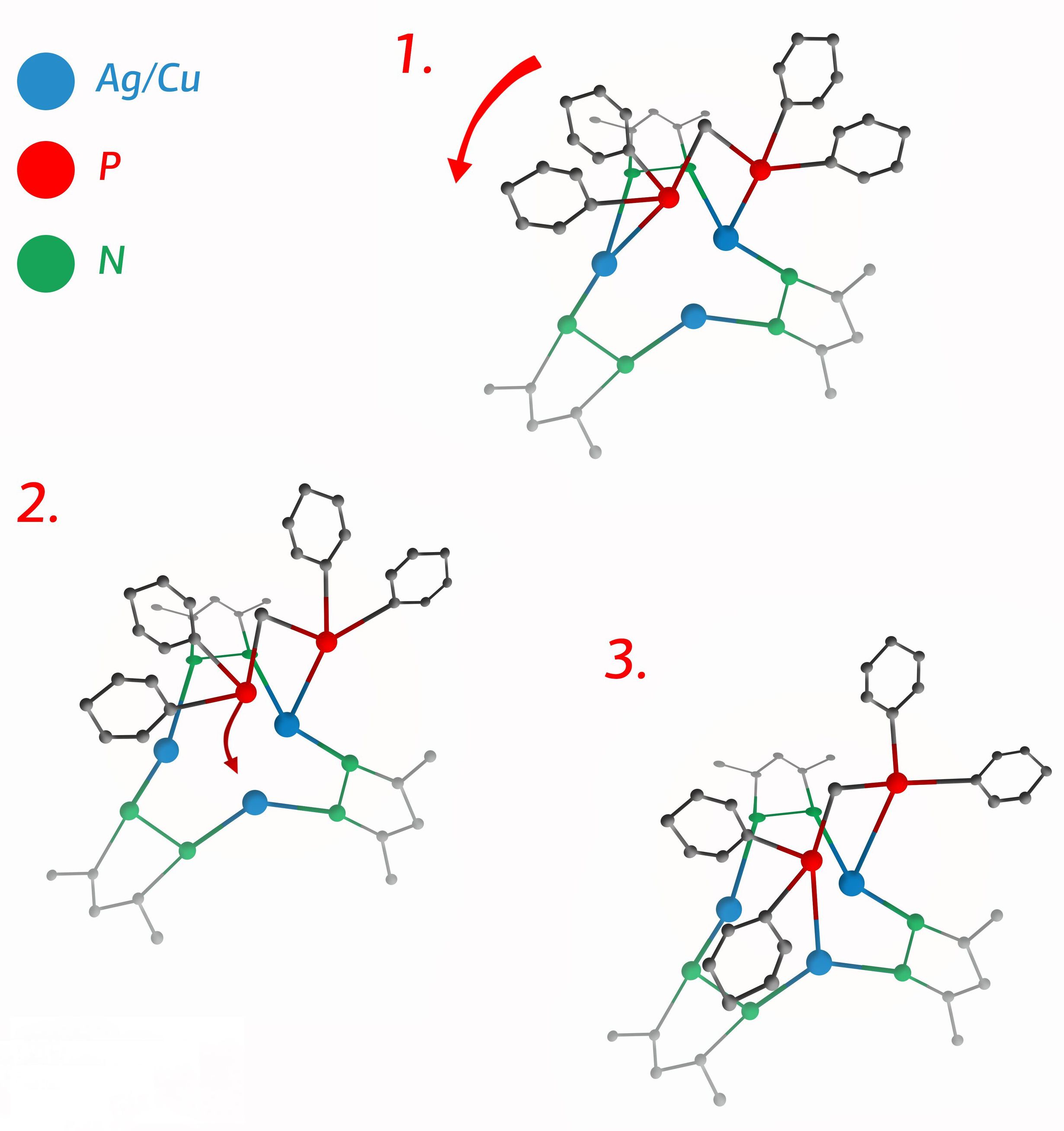 Three configurations of a newly developed “merry-go-round” molecular complex. ) Click for higher resolution
Three configurations of a newly developed “merry-go-round” molecular complex. ) Click for higher resolution
An important feature of the newly developed molecules is that the configuration is stable. That is, the complex does not decay immediately after synthesis as do many other structures of this type.
The researchers determined that the subject merry-go-round structure of complex compounds leads to the emergence of two energy states. Transitions between the two states can produce luminescence. In the case of copper, the structure was found to have a significant quantum yield. More specifically, the ratio of the number of absorbed photons to emitted photons was 41%.
The researchers conclude their article with a statement about the newly developed materials: “now for the first time (we) managed to show a sufficiently high quantum yield on systems that opens up new opportunities for novel OLED displays.” -Arthur Berman