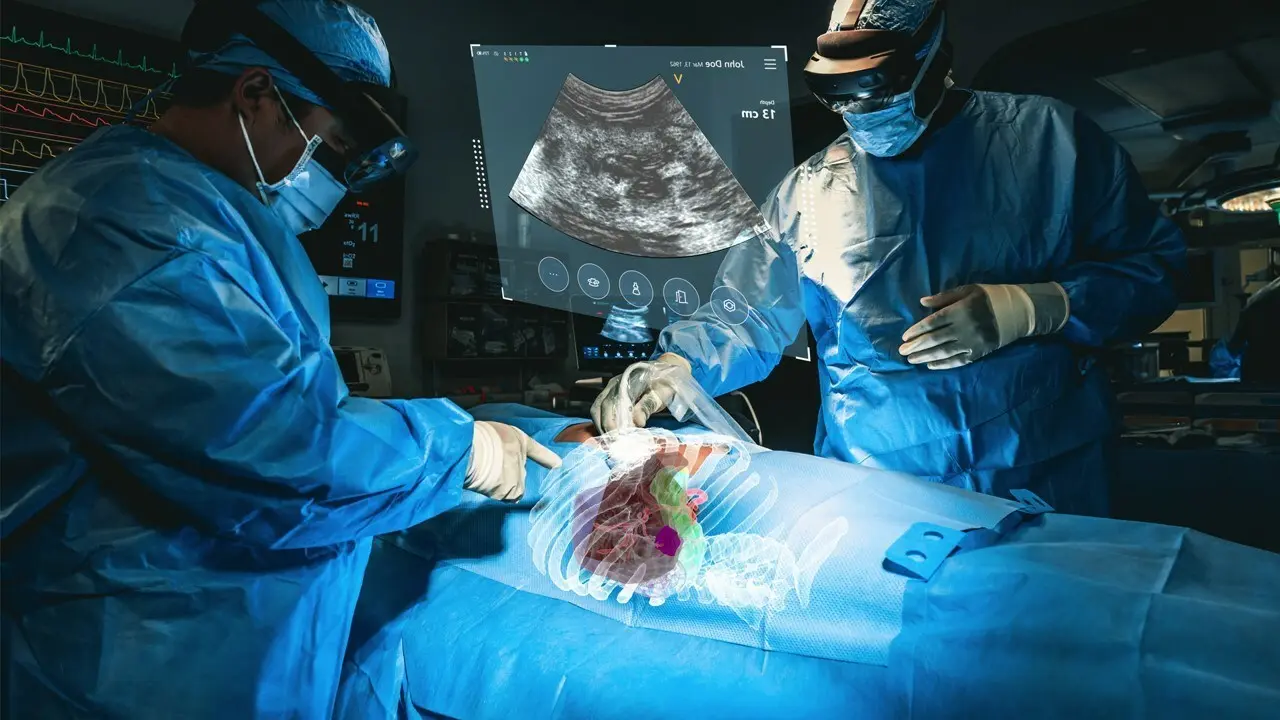
MediView XR Receives FDA Clearance for XR90 Augmented Reality Surgical Platform
by Artem Alekseenko
This is not only the first 510(k) clearance for MediView, but it is the first 510(k) clearance for an augmented reality device utilizing live imaging combined with 3D XR visualization for pre- and intra-operative indications.
Tags:Augmented Reality| Holographic Displays| Medical| MediView XR
