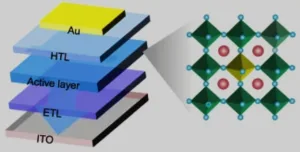
A team of researchers led by Rui Sun of the State Key Laboratory of Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University (Changchun, China) have developed a method to fabricate single component white light emitting LEDs. The approach is based on the use of lanthanide ion doped perovskite nanocrystals.
The up side to perovskite nanocrystal materials, specifically lead halide perovskite nanocrystal materials, is that they can have a high photoluminescence quantum efficiency, an adjustable bandgap, excellent photoelectric properties and that they are easy to fabricate. Based on these properties, LEDs built from such materials would seem to be a good candidate for use as a low cost, solid state light source. To make this potential a reality, it is necessary that such LEDs emit white light. White light perovskite LEDs could, in principle, be created by forming a film composed of a stack of nanocrystals layers. The material in each layer would be slightly different and emit a slightly different wavelength of light that collectively adds up to white light. Unfortunately, up to now, this approach has not been successful. One reason for the failure relates to halide ion segregation and exchange that eventually leads to severe color instability.
Addressing this issue was the focus of the research undertaken by the team. The approach adopted by the team to fabricate single component white light emitting LEDs was based on the doping CsPbCl3 perovskite nanocrystals with lanthanide (Ln3+) ions.
(Note: Lanthanides are the rare earth elements of the periodic table – elements with atomic numbers from 58 to 71 following the element lanthanum. All lanthanide elements form trivalent cations, Ln3+, whose chemistry is largely determined by their ionic radius.)
A recent article by the team on this topic is entitled “Efficient single-component white light emitting diodes enabled by lanthanide ions doped lead halide perovskites via controlling Förster energy transfer and specific defect clearance.” It was published in Light: Science & Applications volume 11, Article number: 340 (2022). A copy of the article is available on-line and can be found below and on Nature.com.
Some specifics of the approach adopted by the team are as follows.
The first step was to dope K+ ions into the CsPbCl3perovskite nanocrystal lattice. This served to tune the perovskite bandgap by partially replacing existing Cs+ ions. The rationale for this approach is that Cs+ ions are well matched to the transition energy of some Ln3+ ions from the ground state to the excited state and that this, in turn, greatly improved the Förster energy transfer efficiency from excitons to Ln3+ ions.
(Note: Förster energy transfer is a mechanism describing energy transfer between two light sensitive molecules or chromophores. A donor chromophore, initially in its electronic excited state, may transfer energy to an acceptor chromophore through nonradiative dipole-dipole coupling. The efficiency of this energy transfer is inversely proportional to the sixth power of the distance between donor and acceptor, making Förster resonance energy transfer extremely sensitive to small changes in distance.)
The next step in the development process was to deal with the problem of efficiency loss associated with spin coating thin films from a colloidal solution – one of the steps used to fabricate the subject LEDs. This issue was addressed by passivating the perovskite nanocrystals with creatine phosphate molecules. Creatine phosphate acts as a tightly bound, surface capping multifunctional ligand and regulates perovskite nanocrystal film formation. Regulation can be used to enhance the optical and electrical properties of the resulting film.
The researchers report that efficient and spectra stable LEDs could be fabricated utilizing active layers made of modified perovskite nanocrystals having various Eu3+ doping concentrations. LED samples prepared with these dopants demonstrated tunable electroluminescence extending from blue to orange.
One of the more specific top level results reported in the article was for a prototype single component white light emitting LED device fabricated utilizing 3.5 mol% Eu3+ doped into K0.15Cs0.85PbCl3 perovskite nanocrystals. These devices showed a peak luminance of 1678 cd/m2 and a maximum external quantum efficiency of 5.4%. The researchers represent these results as being excellent performance for a single chip, white perovskite nanocrystal LED.
In their article, the researchers’ explained that the electroluminescence originated from the defects induced by Ln3+ ions. Going even further, they go on to explain that the method of bandgap modulation and defect passivation that they investigated can be generalized to the doping of other types of Ln3+ ions into perovskite LEDs with the result of similar improved electroluminescence.
The researchers conclude their article with the statement that their work “demonstrates universal strategies in the realization of highly efficient and stable white LEDs via single component Ln3+ ions doped perovskite nanocrystals, which provides an optimal solution for the development of low cost and simple white perovskite LEDs.”