A team of researchers led by Dr. Elton Santos of the School of Mathematics and Physics at Queen’s University (Belfast, Ireland) have discovered that, when Quantum Dots (QDs) are clustered together, they are more fluorescent.
The reason that this effect is important is that greater fluorescence can result in the light emitted by the QDs having a wider range of colors. This, in turn, has the potential to lead to displays that are “brighter, lighter and more energy efficient.”
A recent report on this topic was published by the team in an article entitled “Aggregation-induced emission in lamellar solids of colloidal perovskite quantum wells.” It appeared in Science Advances 22 Dec 2017:Vol. 3, no. 12. A copy of the article is available on-line and can be found here.
In their research, the team investigated QDs containing the material methylammonium lead bromine (MAPbBr3). This material has what is called a perovskite structure. The reader is referred to an article in Wikipedia to learn more about this crystal structure and some of the properties of such materials.
Normally, quantum yield, which determines the brightness of the light emitted from QDs, degrades significantly “as the QDs aggregate, forming crystalline solids.” The team addressed this issue by creating a lamellar structure.
The lamellar structure consisted of thin layers of a so-called perovskite colloidal quantum well material alternating with a long alkyl ligand material. The technical article describes in detail the materials used in the experiments as well as the device fabrication techniques.
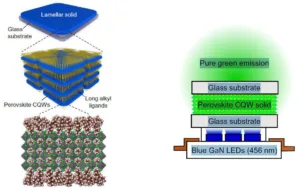
The layered structure produced by the team was a solid material. It is illustrated in the left hand portion of the figure below.
Left: Composition of the perovskite lamellar colloidal quantum well structure. Right: Downconverting light from a blue light emitting diode to produce ultrapure green light.
The layered structure was incorporated into a device that was used to demonstrate ultrapure green light emission. The emission was produced by downconverting light from a blue gallium nitride light emitting diode. The device is illustrated in the right hand portion of the figure above. At room temperature, a luminous efficacy was achieved that was higher than 90 lumen/W at 5000 cd/m². The researchers state that an efficiency this high has not been previously achieved in any other nanomaterial assembly.
The high efficiency is a result of the fact that a large fraction of light that would have normally been absorbed was now being re-emitted. The result was the production of very bright colored light. The team named the new process Aggregation-Induced Emission (AIE).
Dr. Santos explained that
“This AIE process can revolutionize the quality of the colors in TVs because the base colors are red, blue and green. Using AIE we can create the brightest green color ever achieved by any nanomaterial. Once this bright green is integrated with the other two colors, the number of new color combinations could exceed what is currently possible. The latest QD technology, which is just about to be released to market, allows for one billion colors, which is 64 times more than the average TV. However, what using the process we have discovered, we can actually make this even better.”
Another team member, Professor Shangchao Lin of Florida State University, commented that
“Our findings also show that the perovskite nanocrystals emit light extremely quickly and are very energy efficient. This means reduction of electricity consumption, and consistent color expression throughout a long lifespan.”
The researchers are currently looking for similar QD materials and structures for the production of blue and red light. The availability of these colors would make possible the creation of a display that could produce the entire range of colors that can be seen by the human eye.
Team member Professor Shih of ETH-Zurich believes that the technology is almost ready for commercialization. He explained that “The remaining tasks will be to enhance the stability of these compounds and to ensure that they can endure high temperatures, humidity and electrical energy being applied.” -Arthur Berman
Queen’s University, Elton Santos, +44 (0)28-9024-5133 X3643, [email protected]