Perovskite is a mineral that was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and is named after Russian mineralogist L. A. Perovski. Naturally occurring variations of perovskite have been discovered by other mineralogists over the years.

Q: Why is this somewhat obscure mineral of interest to Display Daily readers?
A1: Because variations can produce blue, green, red and IR laser light.
A2: Because it can convert blue light to narrow band green or red light very efficiently.
A3: Because it can make solar cells that are as efficient as silicon solar cells.
A4: Because it is much cheaper to manufacture than GaN or Silicon.
Perovskite, as the word is used today, is not a single mineral, it is a material that has the generic formula ABX3 where A and B represents two different ionic atoms or organic structures and X is most commonly oxygen, although it can also be fluorine, chlorine or iodine. The A atom or structure is always larger than the B atom. A structure that can replace the A atom is the methylammonium cation (CH3NH3+). The natural mineral perovskite discovered by Rose is CaTiO3.
Perovskite is used to describe any material that has the same crystal structure as this naturally occurring CaTiO3. This crystal structure is described as pseudo-Cubic because the unit cell of the idealized material is shaped like a cube. Depending on the A and B atoms or structures and any doping applied to enhance its properties, the cubic cell may be distorted into an orthorhombic shape. In the image of the unit cell, the dark blue spheres are the larger calcium atoms, the red spheres are the oxygen atoms and the light blue sphere partially obscured by the blue-green structure is the smaller titanium atom.
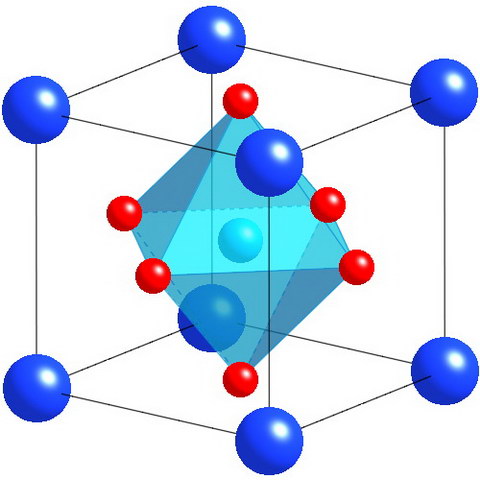 Crystal structure of a representative perovskite
Crystal structure of a representative perovskite
Perhaps the most intense interest in perovskites comes from the solar cell community. One of the advantages of these materials as a generic class is they can be manufactured with high crystal quality by spin coating and solution processing. This simple processing makes them much easier to make commercially than the crystal growing techniques used to make single crystal GaN or Silicon. In terms of large areas, as needed for solar cells, it is also simpler than the vacuum chambers and thermal or laser annealing that is required for HTPS or LTPS. One 2013 estimate of the cost of perovskite solar cells was that they would only cost 20% what thin film silicon solar cells would cost yet they would have the same light-to-electricity conversion efficiency.
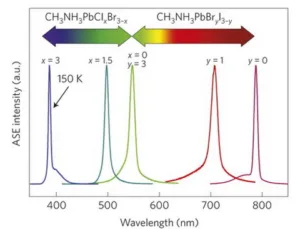
This ability to economically make large area solar cells draws research dollars to perovskites. The display community then benefits from this research: visible lasers and LEDs are, in part, a spin-off of the solar cell research. For example, the abstract of a recent article in Nature Materials (doi:10.1038/nmat4271) titled Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors said “The remarkable performance of lead halide perovskites in solar cells can be attributed to the long carrier lifetimes and low non-radiative recombination rates, the same physical properties that are ideal for semiconductor lasers.” The research was done by ten authors from the University of Wisconsin and Columbia University, with Haiming Zhu & Yongping Fu as lead authors. Depending on the exact composition of the lead halide material, the authors report lasing action at nine different wavelengths from 500nm to 800 nm.
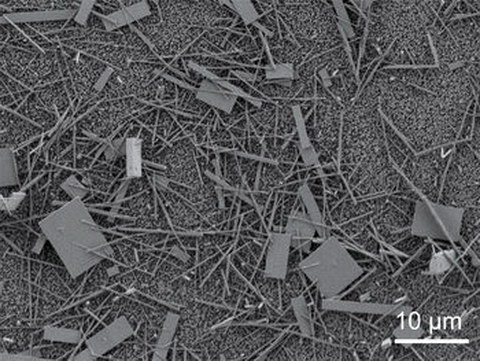 Unsorted nanowire crystals immediately after production. Photo: Song Jin
Unsorted nanowire crystals immediately after production. Photo: Song Jin
Professor Song Jin at the University of Wisconsin, one of the authors of the paper, said, “The single-crystal perovskite nanowires, grown from solutions at room temperature, are high quality, almost free of defects, and they have the nice reflective parallel facets that a laser needs. Most importantly, according to the conventional measures of lasing quality and efficiency, they are real standouts.” The nanolasers were pumped by 402nm blue light, readily available from GaN blue lasers or LEDs, and converted about 70% of the input to laser output. According to Jin, 100% of the photons absorbed by the nanolaser material was re-emitted as laser photons at the new wavelength.
Jin added, “These are simply the best nanowire lasers by all performance criteria, even when compared to materials grown in high temperature and high vacuum. Perovskites are intrinsically good materials for lasing, but when they are grown into high-quality crystals with the proper size and shape, they really shine.”
Wavelengths produced by low-temperature solution-processed lead halide perovskite lasers, as reported in 2014 by researchers at Nanyang Technological University, Singapore (doi:10.1038/nmat3911)
Nanolasers aren’t exactly what the display community wants, they want lasers with watts of optical output, not nanowatts. But this very efficient conversion of light from a blue pump wavelength to a green or red narrow band emission is one thing the display industry is looking for. A quantum dot (QD) will do this by growing the QD to exactly the right size – all QDs in a family have the same material composition and the size of the QD determines the output wavelength. On the other hand, the wavelength output of perovskite nanomaterials is determined by its composition, not its size. It is often easier to control the composition of nanomaterials than the exact size of the nanomaterial specimen. This variation in size is what leads to the relatively broad emission spectrum of QDs compared to lasers.
Research into the optical properties of perovskites for solar cells and displays is exactly that – research – and no perovskite solar cells are currently commercially available. With the pressing need world-wide need for cheap, renewable energy, I expect R&D of these materials will lead to their rapid commercialization. Laser, LED and display products will piggy-back on this solar cell research.
Direct Use in Displays
One commercial application of these solar cells will be applicable directly to display technology. There is an ongoing drive to make wearable electronics “invisible” to the wearer, including wearable displays. Carrying or charging a battery doesn’t count as “invisible.” Since perovskite solar cells can be produced at room temperature with solution processing, they can be produced on flexible substrates. These flexible solar cells can be incorporated into smart systems, like clothing, to provide the needed energy.
Since the need for perovskites to replace QDs is less pressing, the time to commercialization is likely to be longer. Still, I see PeLEDs as a viable alternative to QLEDs in the long term. If I were a QD maker, I’d be looking over my shoulder at these materials. Better yet, I’d be working on their development. –Matthew Brennesholtz