In the same week as Tesla and Panasonic open the Gigafactory – a massive plant in Nevada producing lithium-ion batteries – it is ironic that MIT published a paper on battery evolution, which could make Li-ion devices obsolete.

Published in Nature Energy, the paper deals with so-called lithium-air batteries. The concept is not new: such batteries, which could be used in electric cars and portable electronics, can deliver very high energy output in proportion to their weight. However, in the past drawbacks have included fast degradation and energy wasted as heat, as well as requirements for expensive components and an open-cell design.
MIT has designed a new variation, with a similar theoretical performance to lithium-air batteries but ‘none’ of the drawbacks, and with the ability to be used in a fully-sealed battery. It is called a nanolithia cathode battery.
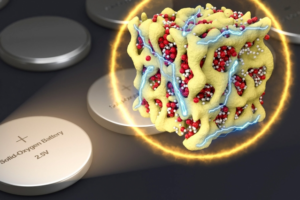
Nanometer-scale particles made of lithium and oxygen compounds (red and white) are embedded in a sponge-like lattice (yellow) of cobalt oxide, which keeps them stable.Conventional lithium-air batteries draw in oxygen from the air. They use this to drive a chemical reaction with the lithium during the discharging cycle, and release it during the charging cycle. In the new battery, the same kind of reaction takes place, but without ever letting the oxygen revert to a gaseous form. Instead, it remains solid and transforms directly between its three redox states, while bound in the form of three different compounds: Li2O, Li2O2, and LiO2. These are mixed together in the form of a glass.
By keeping the oxygen solid, said report author Ju Li, voltage loss is reduced by a factor of five: from 1.2V to 0.24V. Only 8% of the electrical energy is turned into heat, making thermal management less of a concern. Keeping oxygen in its solid form also results in fewer volume changes to the battery, which can disrupt electrical conductivity in its structure.
Li says that the secret to the new formulation is the creation of very, very small particles (nanolithia), at the nanometer scale. These contain both the lithium and oxygen in the form of a glass. This mixture is held tightly in a matrix of cobalt oxide. In this form, the chemical compound transitions can take place entirely within the solid material. Embedding the nanolithia particles inside the cobalt oxide helps to stabilise them and catalyses their transformation.
The new battery has several advantages over conventional lithium-air devices. It does not need to draw in outside air, and so there is no need for the large auxiliary systems that remove carbon dioxide and water from the air. It is also protected from overcharging, because the chemical reaction is ‘naturally self-limiting’. When overcharged, the reaction shifts to a different form that prevents further activity. According to Li, “We have overcharged the battery for 15 days, to a hundred times its capacity, but there was no damage at all.”
In tests, the researchers put a lab version of battery through 120 charge-discharge cycles, with less than a 2% capacity loss. This means that such batteries could have long lifetimes. They could also be adapted to existing installations relatively easily, and can hold as much as double the amount of energy as a conventional lithium-ion battery for a given cathode weight.
The team expects to move from a lab-scale proof of concept to a practical prototype within ‘about a year’.
Editor’s Comment
This article was originally created at the beginning of August. We seemed to have missed the moment, but with all the issues of the Samsung Note 7, I thought it worth including. (BR)