One group of electro-luminescent (EL) devices is known as high-field ELs. The other main group is known as the Light Emitting Diodes, which is itself split into two groups consisting of those devices using inorganic light emitting materials and those using organic light emitting materials.
The devices using inorganic light emitting materials are known simply as LEDs, while those using organic materials are known as OLEDs. The reason that the inorganic group has the same name as the higher level group, rather than being known as ILEDs, is that they were developed before it was known that there were organic materials suitable for creating LEDs and the name has stuck.
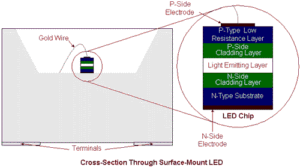
A diode is a device which allows an electric current to flow in one direction only. In an LED, this device is formed by a semiconductor P-N (Positive-Negative) junction, the point at which an abrupt change in the type of impurity within a crystal’s structure occurs, switching from acceptors (p-type) to donors (n-type). When a current is applied across the junction, carriers, in the form of electrons or holes (electrically, the opposite of electrons), are liberated from one side and cross it. Their recombination on the far side causes photons to be released. Some of these are absorbed by the semiconductor material but others escape as light.
In the first LEDs to become commercially available, the semiconductor was made from a combination of three elements: gallium, arsenic and phosphorus (GaAsP). They produced a red light and appeared in the early 1960s. Other materials were investigated and, in the mid 1970s, a combination which produced green light was found. In the late 1980s, a blue-producing compound was developed but the resulting LEDs weren’t very bright and it wasn’t until the mid 1990s that a bright blue LED was developed. At about the same time, new compounds, such as indium gallium aluminum phosphide, were found which produced brighter, more efficient LEDs with lifetimes of around 100,000 hours or 11 years of continuous use.
LEDs have extremely fast switching times (in the order of a few hundred nanoseconds) and, with the arrival of bright blue LEDs, it became possible to create matrices of red, green and blue LED clusters for use as large scale displays, with each cluster addressed as a single pixel. Though the pixel-pitch of such displays are in the region of 10mm, their high brightness, reliability, ruggedness and low power requirement make them ideal for open air displays and they are commonly seen in sporting stadia and at other public events.
Update: Since the article was written, LEDs have become ubiquitous in large displays, traffic signals and automotive lighting. They are increasingly being used in general lighting applications. For some time, LEDs have been used to illuminate the LCDs in mobile devices, but there have been recent developments in the use of LEDs as backlights for large LCDs in notebooks, TVs and monitors. At the time of writing, there are still significant power and cost disadvantages, but LEDs bring the possibility of a wide colour range (gamut). They can also allow the development of field sequential LCDs which could radically affect the cost structure of the LCD industry.
Large area LED displays have also been developed down to a pixel pitch of just 3mm although the cost of such displays is extremely high.