Avatar Medical, a MedTech startup, has developed a virtual reality (VR) surgical planning solution that has recently been approved by the U.S. Food and Drug Administration (FDAA). This is a major milestone as FDA 510(k) clearance demonstrates that the product is as safe and effective as a legally marketed device.
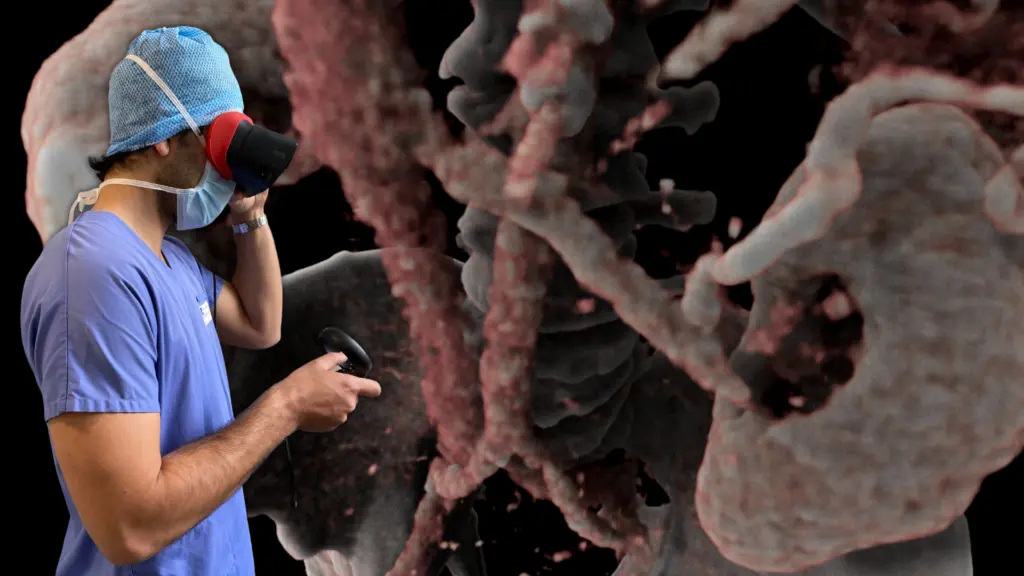
Avatar Medical’s solution helps surgeons better plan their procedures by using VR representations, or avatars, of their patients. These avatars are created in real time from patient CT scans or MRI images using proprietary technology that was developed over four years of research in human-data interaction and machine learning at the Institut Pasteur and Institut Curie.
These avatars are not just helpful for pre-operative planning, they can also be displayed during surgeries. This can provide valuable visual aids for surgeons, potentially increasing the precision and safety of the operations.
The platform has already been used by more than 100 surgeons from 20 different hospitals and universities, including notable institutions such as UMass, CUNY, and Columbia University. It has been utilized for various purposes such as case studies, educating students, and improving patient engagement. This usage has led to 6 medical publications, indicating that the technology has already made a significant impact in the field.
Venkatesh Krishnasamy MD, from Columbia University, comments that the software adds an additional level of capability and confidence to his work in advanced percutaneous and endovascular interventions because it allows him to plan based on preoperative imaging and then verify his work using intraoperative imaging.
Avatar Medical, has announced plans to seek European medical device certification next year.

