In the field of optoelectronics, researchers and scientists continuously strive to develop materials that offer stability and tunable properties. These materials, such as organic-inorganic nanohybrids, have to retain their properties over time and withstand different environmental conditions. Organic-inorganic nanohybrids are made up of organic ligands (small organic molecules) that are bonded to the surface of inorganic nanocrystals. The organic ligands form a protective layer around the inorganic nanocrystal, thereby enhancing its stability.
However, there’s been a challenge: these organic ligands have been known to reduce the conductivity and photon absorption efficiency of the inorganic nanocrystals, somewhat limiting their potential in optoelectronic applications.
In a fascinating turn of events, a research team led by Professor Yoichi Kobayashi from Ritsumeikan University in Japan has demonstrated that these ligands aren’t necessarily fixed and can be displaced under certain conditions. More specifically, they found that exposing an organic-inorganic nanohybrid to visible light leads to what they call a quasi-reversible displacement of the ligands.
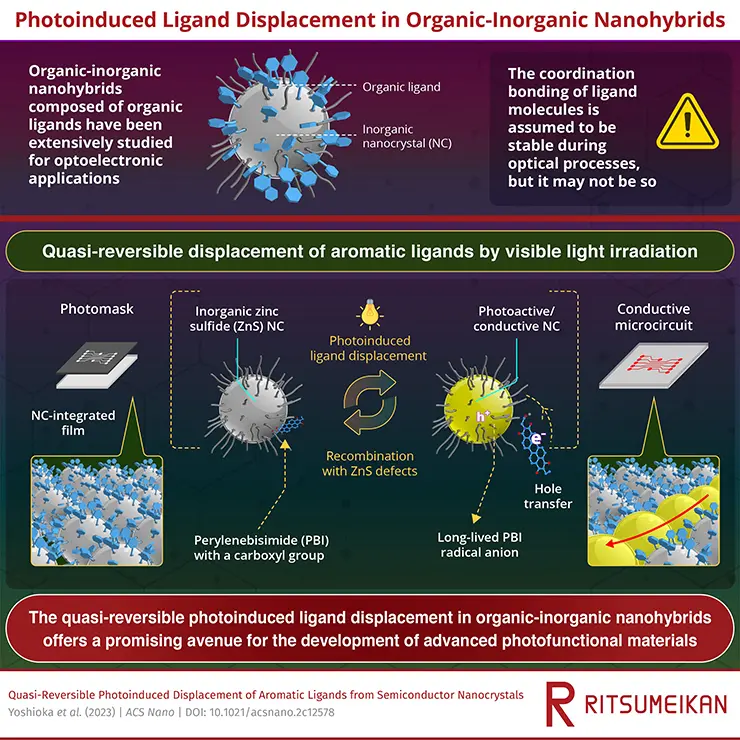
In their study, they used a specific type of nanohybrid that’s composed of perylene bisimide with a carboxyl group (a type of organic ligand, abbreviated as PBI) and zinc sulfide (an inorganic semiconductor, abbreviated as ZnS) nanocrystals. What they observed is that when this PBI-ZnS nanohybrid is exposed to visible light, the PBI ligands get displaced from the ZnS surface. Interestingly, these ligands then gradually recombine with the ZnS nanocrystal surface, making this displacement quasi-reversible.
This discovery is significant because it provides a deeper understanding of how ligands interact with nanocrystals, challenging the previously held belief that ligands are permanently anchored to the nanocrystal surface. In the broader context, this opens up the possibility of developing new photofunctional materials (materials that change properties upon exposure to light) with enhanced functionality.
In layman’s terms, the research could lead to two practical applications:
- Photocatalysts for breaking down tough chemicals: Imagine a material that can harness sunlight to destroy harmful chemicals. This is exactly what a photocatalyst does. It’s a substance that uses light to speed up a chemical reaction. In this case, the researchers are talking about using these photocatalysts to break down chemicals that are usually hard to get rid of, potentially improving waste management and environmental safety.
- Advanced circuitry for wearable devices: Wearable devices like smartwatches and fitness trackers require very thin, flexible circuits. The researchers’ findings could lead to the development of new methods for creating these circuits. Specifically, they’re talking about using light to control the formation of the circuits on the device. This could allow for the creation of more complex, efficient, or reliable circuits in wearable tech, leading to devices that are more powerful, versatile, or easier to produce.
In other words, the ability to control the displacement of ligands on nanocrystals may lead to the development of new, more efficient materials for use in various photofunctional applications. This could bring about significant advancements in the fields of nanoscience, photochemistry, and optoelectronics.
Research
Yoshioka, D., Yoneda, Y., Chang, I.-Y., Kuramochi, H., Hyeon-Deuk, K., & Kobayashi, Y. (2023). Quasi-reversible photoinduced displacement of aromatic ligands from semiconductor nanocrystals. ACS Nano. https://doi.org/10.1021/acsnano.2c12578

