In the development of display technology, a notable advance has been achieved with the creation of electrogenerated chemiluminescence (ECL) cells that exhibit improved levels of luminance and efficiency. This progress is the outcome of research conducted by Associate Professor Takashi Kasahara and master’s student Nanami Ichinohe at Hosei University, together with collaborators from the University of Fukui and National Cheng Kung University. Their work introduces an ECL cell using a yellow phosphorescent iridium complex and a redox mediator to reach new benchmarks in brightness and efficiency for this type of technology.
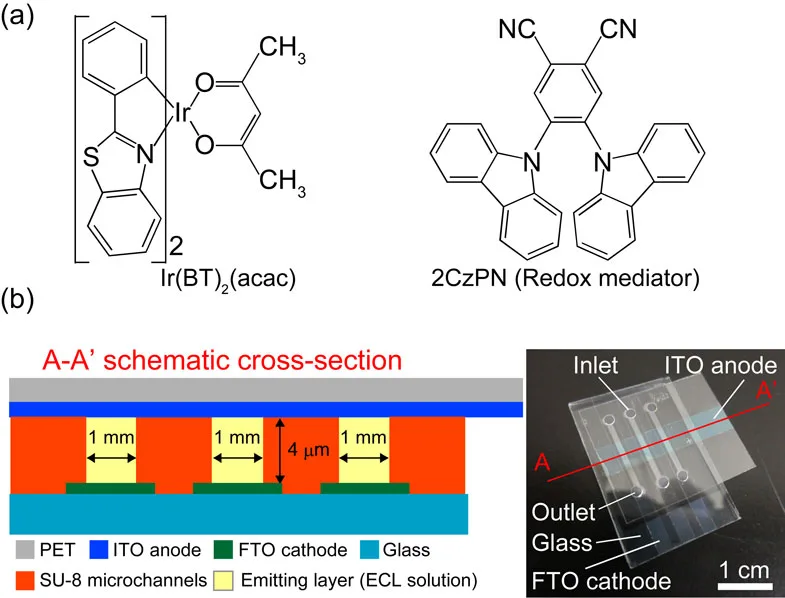
ECL cells are designed to offer advantages in display technology through their simple configuration and manufacturing process. These cells generate light by a process that involves electron transfer reactions between radical ions of the luminescent material, a mechanism that distinguishes them from other self-emissive devices like LEDs and organic LEDs.
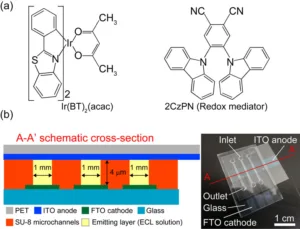
The decision to use iridium complexes in these cells draws on their effectiveness in organic LEDs, where they facilitate room-temperature phosphorescent emissions. The flexibility in changing emission colors by altering ligands adds versatility to their application. In this study, the researchers moved beyond traditional approaches by combining two luminescent materials in a solution, aiming to enhance the cell’s luminance and efficiency.
Dr. Kasahara’s work suggests a path forward for research into ECL systems, indicating potential for the development of efficient, solution-based, self-emissive displays in the future based on the processes being developed, if not the research materials.
Reference
Ichinohe, N., Otsuka, R., Ishimatsu, R., Kobayashi, M., Mizuno, J., Akino, N., & Kasahara, T. (2024). Yellow Phosphorescent Electrogenerated Chemiluminescence Cell Based on a Cyclometalated Iridium Complex with a Redox Mediator. Electrochemistry, 92(2), 027004–027004. https://doi.org/10.5796/electrochemistry.23-00147